Due to multiple immunoregulatory functions in mediating human health and disease, defensins -- the antimicrobial peptides of innate immunity (pdf) -- play a critical role (“Guardians of the Gut”) in maintaining homeostasis in the gastrointestinal (GI) tract.
Deficient Defensin and Mucin Expression in IBD
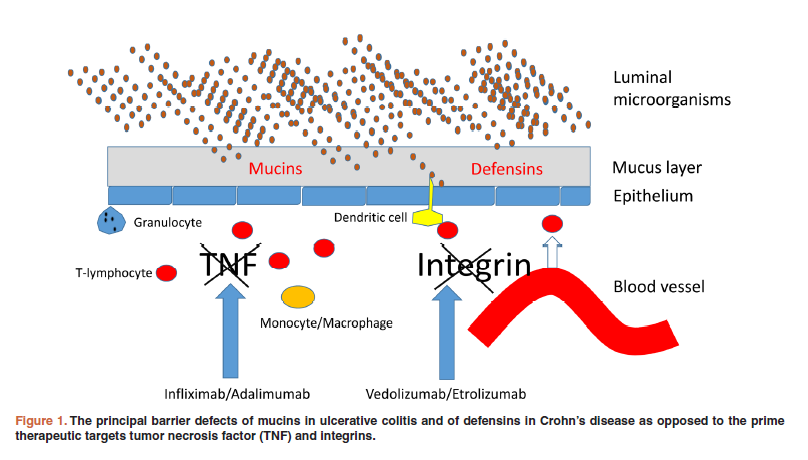
Research suggests (pdf) deficient or impaired defensin expression contributes to the pathogenesis of Crohn’s Disease. The body’s natural barrier (its first line of defense) at the mucosal layer, an innate reservoir for antimicrobial peptides (pdf), becomes compromised, placing it at increased risk of infection and inflammation.
Defects in mucins -- structurally diverse glycoproteins found in the secretions of mucous membranes, e.g., in the GI tract -- also is implicated as a factor in the pathogenesis of Ulcerative Colitis (UC).
(See pages 18-19 of this document, pdf, which includes a discussion with Professor E.F. Stange, an academic expert in defensins' role in IBD. The interview was conducted at the Falk Foundation Symposium on “Evolving Therapies in Clinical Practice in IBD,” held in Prague, April 29-30, 2016.)
The Need for Novel Non-Biologic IBD Treatments
Biologics, a “driving force” in Pharma (comprising 6 of the top 8 drugs by revenue in 2016), have become a welcome treatment option in managing IBD, a complex and costly disease affecting millions of people (worldwide incidence and prevalence). But biologic treatments, often accompanied by initial uptake failures, a diminishing response over time, as well as adverse side-effects on top of a less convenient means of administration, have proven only moderately effective -- the result of targeting pro-inflammatory cytokines, which research suggests largely play a role in later stages of IBD, less so at disease onset.
Moreover, IBD no longer is thought of as a classic autoimmune disease -- instead, primarily as a complicated barrier disorder. A considerable medical need exists for alternative targeted treatments that may act earlier, and intervene more directly, in the pathogenesis of IBD, thereby reducing disease severity and duration, as well as mitigating potential complications.
Defensin-Based Therapeutics in IBD
Brilacidin, modeled (pdf) on defensins, has been shown not only to reduce pro-inflammatory cytokines and chemokines, alongside exhibiting robust antimicrobial activity (see the images at bottom below), but also to inhibit PDE4, which is being advanced as a novel therapeutic approach in the treatment of IBD.
Moreover, per the research cited above -- on the importance of complementary microbe-host interactions in the GI mucosa -- Brilacidin might also benefit from an additional ability to restore defective defensin expression and induce mucin production, strengthening mucosal immunity and integrity. Evidence of mucosal healing (Endoscopic Response) has taken on extra importance as an IBD efficacy measure toward obtaining regulatory approval.
Given unique and broad mechanisms of action, defensin-based therapeutics, of which Brilacidin is a leading example, increasingly are viewed as promising avenues for IBD drug development:
· “A promising approach is the development of novel drugs like defensin-derived molecules that substitute for the missing endogenous antibacterials.” (Source)
· “The potential for enteric defensins as tools of clinical intervention grows as our understanding of their role in host health and immunity increases.” (Source)
· “Knowledge about defensins and others antimicrobial peptides could have a substantial influence on future therapeutic strategies.” (Source, pdf)
(Also see “Defensins, Lectins, Mucins and Secretory Immunoglobulin A: Microbe-Binding Biomolecules that Contribute to Mucosal Immunity in the Human Gut.” Crit Rev Biochem Mol Biol. 2017 Feb; 52(1): 45-56.) (pdf)
Brilacidin for the Treatment of IBD
Innovation Pharmaceuticals has completed a Phase 2 Proof-of-Concept clinical trial evaluating Brilacidin for the treatment of mild-to-moderate Ulcerative Proctitis/Ulcerative Proctosigmoiditis (UP/UPS), two types of IBD. As presented at the 2017 Drug Discovery and Therapy World Congress (click here for pdf of slide deck), a majority of patients treated with Brilacidin achieved Induction of Remission, including Endoscopic Response.
Brilacidin is being developed as a novel, non-corticosteroid, non-biologic IBD treatment, with formulation development plans including oral tablets for the treatment of UC and Crohn's and foam and/or gel for the treatment of UP/UPS.
Source:
“Brilacidin, Host Defense Peptide Mimetic, One of a New Class of Immunomodulatory Agents That Can Target Multiple Disease Indications” (ECCMID 2015) (pdf)