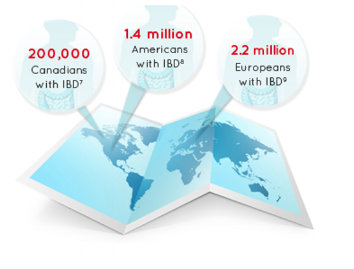
According to a February 2016 Bioworld article, the pharmaceutical industry increasingly is looking for novel treatments (e.g., see "Inflammatory Bowel Disease 2016: What's in the Pipeline?", pdf), in Inflammatory Bowel Disease (IBD) -- a debilitating, hard-to-treat condition that is on the rise, world-wide, a global disease with an accelerating incidence.
Renewed interest in the Gastrointestinal market sector, estimated to grow from $35.7 billion in 2015 to $48.4 billion by 2022, is based on emerging non-corticosteroid and non-biologic therapies: “Although TNF alpha inhibitors still lead the list of clinical trials for drugs targeting IBD, UC and Crohn's, and glucocorticoid agonists appear in the top 10, biopharmas are quickly moving on to other mechanisms.”
The Bioworld story continues: “Many in the field cited a new spirit of openness about GI disorders for bringing deserved attention to the unmet medical need in the space.”
Innovation Pharmaceuticals is planning to develop additional formulations -- such as foam/gel in Ulcerative Colitis, and pill in Crohn's -- for Brilacidin, its first-in-class immunomodulatory drug candidate. Brilacidin is being evaluated in a Phase 2, open-label, proof-of-concept, dose-escalation clinical trial in Ulcerative Proctitis / Ulcerative Proctosigmoiditis (UP/UPS), two types of IBD.
Previously released interim results, through the first two cohorts of patients treated, support Brilacidin's potential in IBD.
The Company completed the final Brilacidin UP/UPS patient cohort in June, with topline results across all three cohorts to be presented at the Drug Discovery & Therapy World Congress in Boston, MA, the afternoon of July 13, 2017.
“For those of us who have been involved in this trial from the start, it is exciting to see Brilacidin perform as well as we thought it would. The endoscopic evidence we have now gathered on patients is particularly telling, Brilacidin’s unique multi-pronged ability to reduce inflammation, fight infection and heal GI tract mucosa has been impressive, based both on subjective and objective scoring measures. It is no surprise to us why we have generated significant excitement regarding Brilacidin among patients, health care professionals and potential industry partners interested in this truly novel IBD drug candidate. ”