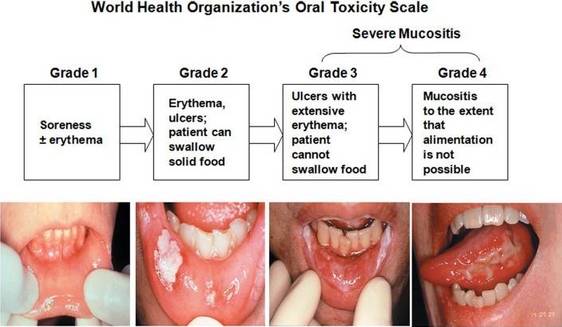
While therapeutic advances are underway, radiation-induced Oral Mucositis (OM) remains “one of the most dominant, serious, disruptive, and symptomatic complications of radiation therapy used for the treatment of cancers of the head and neck.”
Affecting approximately 500,000 people in the United States, OM occurs in 100 percent of altered fractionation radiotherapy head and neck cancer patients, resulting in: interruptions to cancer therapy (dose reductions or delays, video); poor local tumor control; and added treatment costs of up to $25,000 per patient. Sixty-two percent of OM sufferers require hospitalization and 70 percent with Grade 3 or Grade 4 OM require feeding tube insertion. This painful and debilitating condition, in its severest forms, can become life-threatening as food and water intake, by patients, is compromised.
In December 2004, the FDA approved IV-administered Kepivance (Palifermin) for the treatment of OM (see the FDA Approval Package, pdf). Approval was based primarily on the results of a randomized, placebo-controlled, multicenter trial in 212 patients. In the primary efficacy study, the median duration of WHO grade 3 and 4 OM was shorter (three days vs. nine days) in patients receiving palifermin.
As noted in a prior blog post, the Company is testing Brilacidin, its immunomodulatory drug candidate, in a Phase 2 multi-center, randomized, double-blind, and placebo-controlled study (see NCT02324335).
The Brilacidin-OM trial has completed full patient enrollment, having earlier achieved highly encouraging interim results. Severe OM was experienced by only 22.2 percent of Brilacidin-treated patients compared to 70 percent in the placebo group. This suggests Brilacidin has strong potential in preventing severe OM.
Brilacidin’s dual properties, as an anti-inflammatory and as an anti-bacterial agent, may make it particularly well-suited for treating OM, and potentially Gastrointestinal Mucositis (GIM) and mucositis in the rectal area, given common features in their pathogenesis (see “New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury” in Front. Pharmacology, 08 June 2017). Many of the cytokines and chemokines amplified in OM, such as TNF-α and IL-1β, have been shown to be favorably mitigated by Brilacidin due to its unique immunomodulatory profile and broad mechanism of action.
Dr. Stephen Sonis, DMD, DMSc, of Dana-Farber Cancer Institute and an expert in OM, having authored numerous publications and journal articles, is consulting with the Company (a member of its Scientific Advisory Board) in the development of Brilacidin as a possible preventative treatment for severe OM.
Brilacidin has been granted Fast Track by the FDA, which makes it eligible for more frequent FDA meetings, as well as potentially accelerated approval and priority review. The Company also plans to submit Brilacidin for FDA Breakthrough Therapy designation in the treatment of OM if results similar to those observed, at interim, continue to be seen through the conclusion of the study, planned to be completed in 4Q2017.