Innovation Pharmaceuticals and George Mason University’s National Center for Biodefense and Infectious Diseases (NCBID) have completed extensive pre-clinical laboratory testing demonstrating the potent anti-SARS-CoV-2 activity of Brilacidin, a defensin-mimetic drug candidate, which is being developed as a potential COVID-19 treatment.
Research findings are being submitted for peer-review publication. The preprint can now be downloaded on bioRxiv.org at the link below. Preprint highlights are also excerpted below.
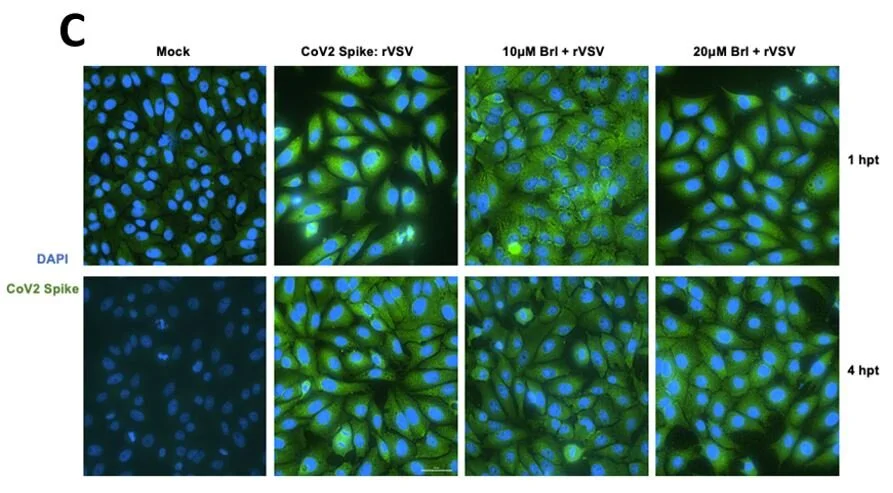
Brilacidin, a COVID-19 Drug Candidate, Exhibits Potent In Vitro Antiviral Activity Against SARS-CoV-2
https://www.biorxiv.org/content/10.1101/2020.10.29.352450v1.full (pdf)
“In this manuscript, we demonstrate brilacidin exhibits robust inhibition of SARS-CoV-2 in Vero cells and Calu-3 cells, supporting brilacidin as a promising novel drug candidate for the treatment of COVID-19. Functioning as a viral entry inhibitor, proposed mechanisms of action for brilacidin include affecting the integrity of the viral membrane and preventing viral binding to cells. More detailed mechanistic studies are planned. Destabilizing viral integrity is a desirable antiviral property, especially in relation to pan-coronavirus agents, as the viral membrane is highly conserved and similar in construct across different coronavirus strains. Moreover, drugs that can directly disrupt viral integrity would be less prone to resistance due to mutation, unlike many antivirals, antibody-based treatments and vaccines that are currently in use and in development. Brilacidin exhibits an excellent synergistic inhibitory profile against SARS-CoV-2 in combination with remdesivir. Experiments conducted in endemic human coronaviruses are ongoing, with additional testing planned in other lethal coronaviruses (MERS-CoV, SARS-CoV), toward assessing the potential of brilacidin as a broad spectrum inhibitor of coronaviruses.”
Source: Bakovic, A., et al. (2020). “Brilacidin, a COVID-19 Drug Candidate, Exhibits Potent In Vitro Antiviral Activity Against SARS-CoV-2” (preprint) (pdf)