SHAREHOLDER ALERT
In news released on Tuesday, the Company announced that in vitro research being independently conducted at a U.S. Regional Biocontainment Laboratory (RBL) revealed Brilacidin reduced the viral titer (load) of SARS-CoV-2, the novel coronavirus responsible for COVID-19, by 75 percent as compared to vehicle control after only 1 hour of preincubation prior to infection. The concentration of Brilacidin tested was 10μM.
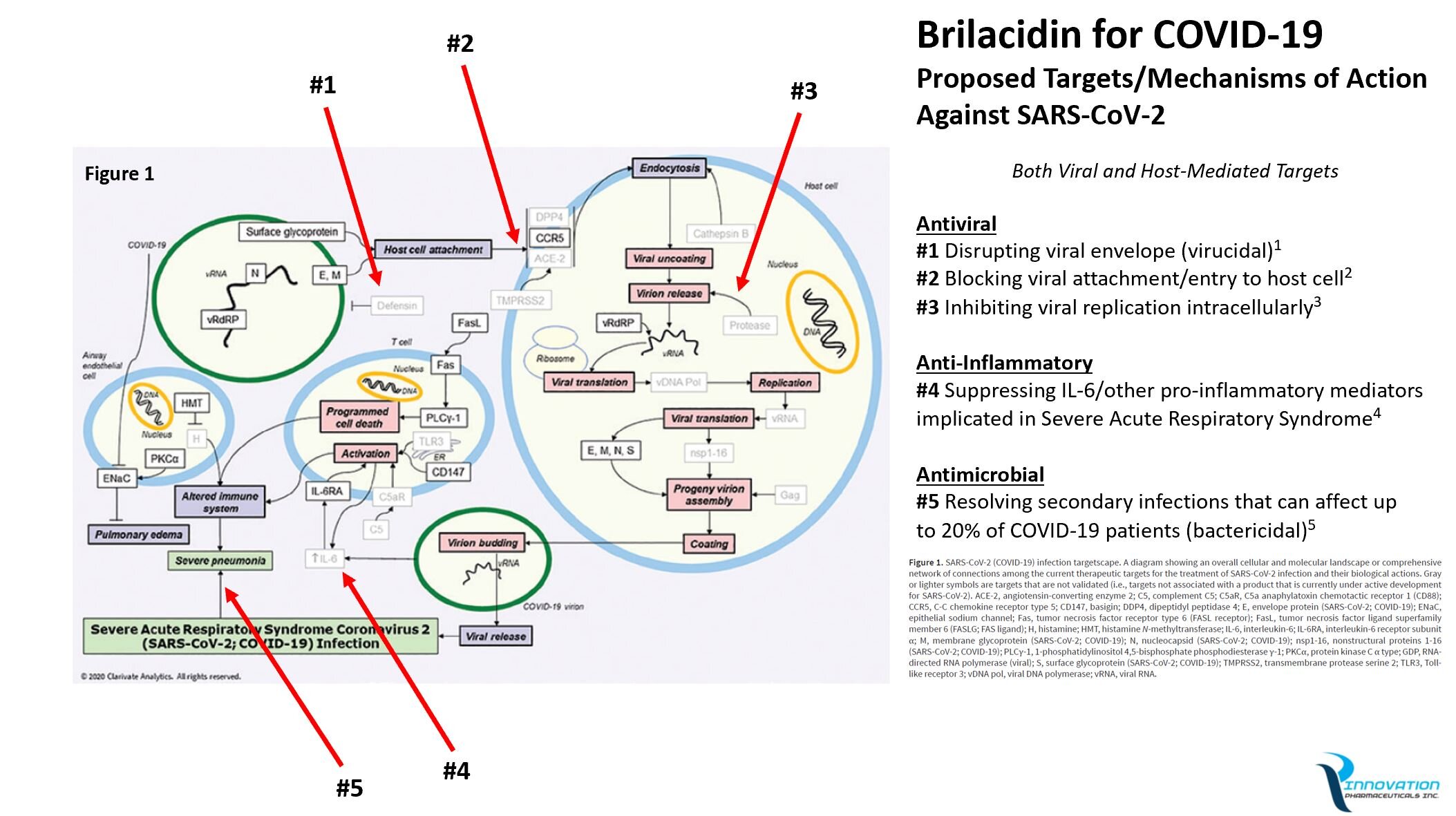
Brilacidin’s potent virucidal ability to rapidly inactivate (essentially “kill”) the novel coronavirus upon contact distinguishes it from all other known anti-SARS-CoV-2 drug candidates in development. Gilead’s remdesivir, along with other antivirals being repurposed to treat SARS-CoV-2 (e.g., lopinavir, ritonavir, favipiravir, etc.), act only to prevent viral replication once host cells have already been infected by the novel coronavirus. Whereas Brilacidin, prior to infection, has the potential both to disrupt the virus directly and prevent viral entry into host cells, in addition to inhibiting viral replication post-infection—making for three distinct potential direct antiviral mechanisms of action.
Below is information and a graphic that illustrates how Brilacidin is well-positioned to emerge as a possible breakthrough drug and the gold standard for treating coronaviruses and potentially other viral diseases, given its multiple antiviral and other complementary therapeutic properties.
Proposed Targets/Mechanisms of Action of Brilacidin Against SARS-CoV-2
1) Antiviral—Virucidal Property (pre-infection, disrupting the viral envelope/destroying the virus)
2) Antiviral—Blocking Property (pre-infection, preventing viral attachment/viral entry to host cells)
3) Antiviral—Inhibitory Property (post-infection, interfering with viral replication intracellularly)
4) Anti-Inflammatory Property (post-infection, suppressing IL-6 and other pro-inflammatory mediators implicated in Severe Acute Respiratory Syndrome)
5) Anti-Bacterial Property (post-infection, helping to resolve secondary infections that can affect up to 20 percent of COVID-19 patients)
In a previous Phase 2b clinical trial, Brilacidin was proven to have potent bactericidal properties against difficult Gram-positive Infections (ABSSSI), including methicillin-resistant Staph aureus (MRSA). (See above #5).
Exhibiting multiple antiviral mechanisms of action and other complementary therapeutic properties would likely greatly enhance Brilacidin’s efficacy against SARS-CoV-2 (COVID-19) while decreasing the chances of mutational-driven resistance to the drug.
Research and evaluation of Brilacidin against SARS-CoV-2 using both VERO and human cells continues at the RBL. The Company’s expects to release data on multiple studies over the coming weeks.
Adapted from: Sorbera LA, et al. “Taking Aim at Fast-Moving Target: Targets to Watch for SARS-CoV-2 and COVID-19” (pdf). Drugs of the Future, 45(4):1-6 (Advanced Publication). Clarivate Analytics.
1 See May 19, 2020 Press Release
2 See May 26, 2020 Press Release; Wang C, et al (2020); Whisenant J and K Burgess (2020); and Kit O and Y Kit (2020)
3 See Cavasotto C and JD Filippo (2020); Pant S, et al (2020)
4 Data on file
5 Date on file
Forward-Looking Statements: This shareholder alert contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 including statements concerning the future execution of a definitive agreement with a global pharmaceutical company and the anticipated terms thereof, our future drug development plans, other statements regarding future product developments, and markets, including with respect to specific indications, and any other statements which are other than statements of historical fact. These statements involve risks, uncertainties and assumptions that could cause the Company’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward-looking statements. The Company has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are the Company’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; including the amount and timing of the sale of shares of common stock under securities purchase agreements; the fact that the Company’s licensee(s) may not successfully complete pre-clinical or clinical testing and the Company will not receive milestone payments, or the fact that the Company’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. A more complete description of these risk factors is included in the Company’s filings with the Securities and Exchange Commission. You should not place undue reliance on any forward-looking statements. The Company undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.