Innovation Pharmaceuticals Data from Phase 2 Brilacidin Oral Mucositis (OM) Trial in Head and Neck Cancer Show Notable Reductions in Median Duration of Severe OM and in Number of Unplanned Visits/Hospital Admissions Due to OM
· Median Duration of Severe Oral Mucositis (SOM) Reduced to Less Than One Day
· No Unplanned Office Visits, Emergency Department Visits, and/or Hospital Admissions Due to OM in Brilacidin-OM Arm vs. Four in Placebo Arm
· Data Align with Previously Reported 80.3% Risk Reduction in Incidence of Severe OM in Per Protocol Population Receiving Aggressive Chemotherapy Regimen Compared to Placebo
BEVERLY, MA – April 16, 2018 (GLOBE NEWSWIRE) Innovation Pharmaceuticals (OTCQB:IPIX) (“the Company”), a clinical stage biopharmaceutical company, is pleased to report additional information from the Company’s successfully completed Phase 2 clinical trial of Brilacidin-OM (see NCT02324335) for the indication of decreasing the incidence of Severe Oral Mucositis (SOM) (WHO Grade ≥3) in Head and Neck Cancer (HNC) patients receiving chemoradiation.
These additional data align with previously released Brilacidin-OM results showing a risk reduction in the incidence of SOM, including up to an 80.3% risk reduction in the incidence of SOM among patients receiving more aggressive chemotherapy. Other previously released results indicate Brilacidin-OM also delayed onset of SOM. The Company is developing Brilacidin-OM under FDA Fast Track designation as a convenient, and clearly differentiated, therapy aimed to decrease incidence of SOM.
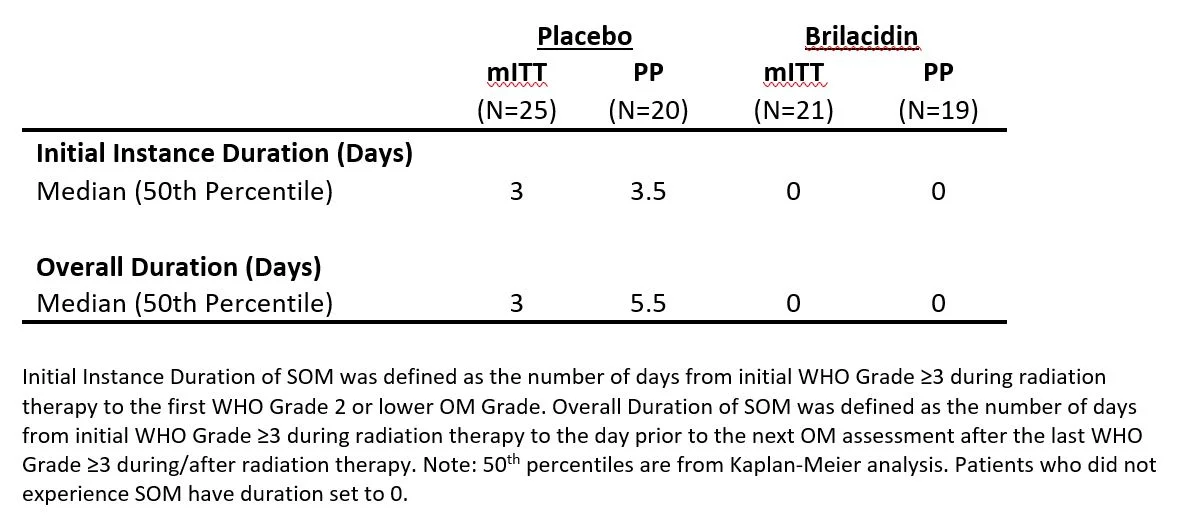
Secondary Endpoint: Duration of SOM
Brilacidin outperformed placebo in both the Initial Instance Duration of SOM and the Overall Duration of SOM, as shown below.
Exploratory Endpoint: Unplanned Office Visits, Emergency Department Visits, and/or Hospital Admissions Due to OM
Positive OM assessment endpoints are additionally supported by zero (0) of the patients in the Brilacidin-OM group having unplanned office visits, ED visits, or hospital admissions due to OM, compared to four (4) patients in the placebo group.
Other Study Observations
· Regardless of the oral sites irradiated (at least two sites from: buccal mucosa, floor of mouth, ventral/lateral tongue, and soft palate), the incidence by patient of Severe OM on Brilacidin-OM relative to placebo was consistently reduced.
· Across cumulative radiation dose intervals, patients in the Brilacidin-OM group consistently reported less often feeling the sensation “swollen” (approximately half of that reported for the placebo group). “Burning” sensation also was reported consistently less frequently in the Brilacidin treatment group.
· Patients in the Brilacidin-OM group appeared to trend more favorably over the course of chemoradiation treatment according to Eastern Cooperative Oncology Group (ECOG) Performance Status—a common set of criteria used in oncology trials to assess debility.
Management Comments
“Drug makers, the world-over, have spent decades and enormous sums in both money and resources trying to develop an effective OM drug in a bid to address dire patient needs as well as capture a tremendous market opportunity,” commented Leo Ehrlich, Chief Executive Officer at Innovation Pharmaceuticals. “Yet, there is currently no drug approved to treat, let alone prevent, severe OM in patients with Head and Neck Cancer. Most Pharmas currently conducting OM trials target shortening the duration of severe OM as their primary endpoint, not reduction of incidence, like we did. The Brilacidin-OM Phase 2 trial met its primary objective and its key secondary objectives. As we continue to analyze subset data, we are extremely enthusiastic about observed trends. Hundreds of thousands of patients would benefit from a preventative OM treatment and we’re excited that Brilacidin-OM may one day provide these patients a much-needed breakthrough treatment option.”